 |
Sample preparation techniques for electron microscopy |

|
This page brings together all of the resources that use sample preparation techniques at low temperature for a better morphological preservation. The samples thus prepared can be observed by transmission (TEM) or scanning (SEM) cryomicroscopy.
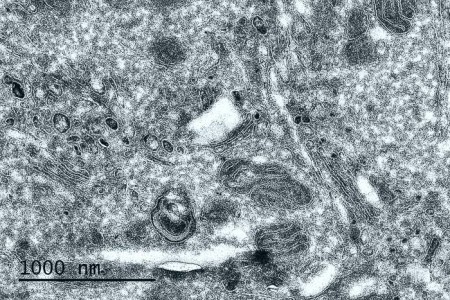
Cryo-section of chemically fixed tumor cells (Tokuyasu technique) – Observation at 80 KV
Services:
- Ultra-rapid freezing by immersion in liquid ethane, or by high pressure freezing of whole samples (bacteria, viruses, particles, liposomes, etc.) in vitreous ice up to 200 µm in thickness
- Cryo-sections (CEMOVIS and Tokuyasu techniques)
- Automated cryo-substitution and resin embedding at very low temperatures
- Cryo-fracture and sublimation
- Transmission and scanning cryomicroscopy
Access mode:
- With assistance
- As service delivery
Available resources in sample preparation for electron microscopy
Nom |
Technical characteristics |
Location |
| Cryo-ultramicrotome Leica UC7/FC7 |
Removable cryo chamber, cryosphere (to avoid contamination by frost), antistatic system & micromanipulator | METi – UPS Campus |
| Freeze substitution system AFS-2/FSP |
Fully automated processes, associated with an automated device to manage reagents | METi – UPS Campus |
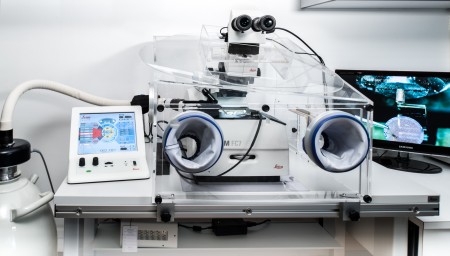
| Cryofixateur high pressure cryo-fixation system Leica EM ICE |
Freezing at -8000 to -12,000°/s under 2000 bars of pressure | METi – UPS Campus |
| Cryoplunge Leica EMGP |
Automated device for ultra-rapid freezing in ethane, equipped with a stereomicroscope | METi – UPS Campus |
| Cryopreparation module PP3000T Quorum |
Cryo transfer chamber for MEB FEG Quanta 250 FEI for metallization, cryo-fracture and sublimation |
CMEAB, Rangueil faculty of medecine |
Some publications made thanks to these resources:
- Bidimensional lamellar assembly by coordination of peptidic homopolymers to platinum nanoparticles. Nat Commun., 11(1):2051. Manai G., Houimel H., Rigoulet M., Gillet A., Fazzini PF., Ibarra A., Balor S., Roblin P., Esvan J., Coppel Y., Chaudret B., Bonduelle C., Tricard S. (2020)
- Good Vibrations: Structural Remodeling of Maturing Yeast Pre-40S Ribosomal Particles Followed by Cryo-Electron Microscopy. Molecules. 25(5):1125. Shayan R., Rinaldi D., Larburu N., Plassart L., Balor S., Bouyssié D., Lebaron S., Marcoux J., Gleizes PE., Plisson-Chastang C. (2020)
- Post-mitotic dynamics of pre-nucleolar bodies is driven by pre-rRNA processing. J. Cell Science Octobre 2012. Carron C, Balor S, Delavoie F, Plisson-Chastang C, Faubladier M, Gleizes PE, O’Donohue MF
- Cord factor (trehalose 6,6′-dimycolate) forms fully stable and non-permeable lipid bilayers required for a functional outer membrane. Biochim Biophys Acta. Sep. 2013. Rath P, Saurel O, Czaplicki G, Tropis M, Daffé M, Ghazi A, Demange P, Milon A.
- Artificial feeding of Varroa destructor through a chitosan membrane: a tool for studying the host-microparasite relationship. Tabart J1, Colin ME, Carayon JL, Tene N, Payre B, Vetillard A. Exp Appl Acarol, 2013,61(1), 107-18.


